Research
Introduction
Webex Link: https://uichicago.webex.com/meet/zhpeng
Principle Investigator: Center for Bioinformatics and Quantitative Biology
Research Interests: Multiscale Modeling; Biomechanics and Biophysics of Cells, Organelles, Molecules, and Tissues; Modeling of Microfluidics, Nanofluidics, and Finite-Reynolds-Number Flows.
My research area is computational science and engineering. My research directions include: 1) Multiscale modeling, such as coupling of continuum-based macroscale modeling with particle-based mesoscale/microscale simulations; 2) Biomechanics and biophysics of cells/organelles/molecules/tissues, such as red blood cells, primary cilia, nuclei, cytoskeletal proteins, DNA/RNA, blood vessel walls; 3) Modeling of microfluidics/nanofluidics, such as acoustic microfluidics, inertial microfluidics, and nanopores. Specifically, an important goal of my group is to apply multiscale modeling to predict how proteins mutations and modifications affect the biomechanics and mechanobiology of cells and tissues within in vitro and in vivo microenvironments. This will help understand the mechanisms of related diseases such as hematologic disorders, ciliopathies, laminopathies, malaria, and cancer metastasis. In pursuit of this goal, the objective of my group is to integrate particle-based simulations such as all-atom molecular dynamics (MD) and coarse-grained dissipative particle dynamics (DPD) with continuum-based modeling approaches such as finite element method (FEM) and boundary element method (BEM) to model the cell-microenvironment interactions starting from the molecular scales. Furthermore, my group is also applying multiscale modeling to help develop deformation-based, acoustic-based, inertial-based, thermal-based, and electric-based microfluidics/nanofluidics for disease diagnostics and biotechnologies. Finally, I also studied several types of bio-inspired structures, including a flow energy harvester of flapping foils inspired by fish swimming, natural/artificial nacre shells, and tensegrity structures.
Red blood cell: modeling of a cell
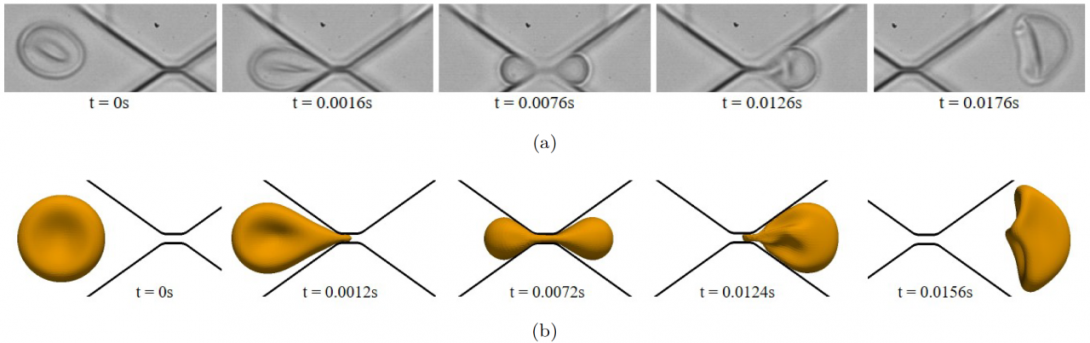
Red blood cells (RBCs) can be cleared from circulation when alterations in their size, shape, and deformability are detected. This function is modulated by the spleen-specific structure of the interendothelial slit (IES). Here, we present a unique physiological framework for development of prognostic markers in RBC diseases by quantifying biophysical limits for RBCs to pass through the IES, using computational simulations based on dissipative particle dynamics. The results show that the spleen selects RBCs for continued circulation based on their geometry, consistent with prior in vivo observations. A companion analysis provides critical bounds relating surface area and volume for healthy RBCs beyond which the RBCs fail the “physical fitness test” to pass through the IES, supporting independent experiments. Our results suggest that the spleen plays an important role in determining distributions of size and shape of healthy RBCs. Because mechanical retention of infected RBC impacts malaria pathogenesis, we studied key biophysical parameters for RBCs infected with Plasmodium falciparum as they cross the IES. In agreement with experimental results, surface area loss of an infected RBC is found to be a more important determinant of splenic retention than its membrane stiffness. The simulations provide insights into the effects of pressure gradient across the IES on RBC retention. By providing quantitative biophysical limits for RBCs to pass through the IES, the narrowest circulatory bottleneck in the spleen, our results offer a broad approach for developing quantitative markers for diseases such as hereditary spherocytosis, thalassemia, and malaria. MIT news
Igor V. Pivkin*, Zhangli Peng*, George Em Karniadakis, Pierre A Buffet, Ming Dao, and Subra Suresh. Biomechanics of red blood cells in human spleen and consequences for physiology and disease. Proceedings of the National Academy of Sciences of the U.S.A.,, 2016. (* contributed equally).
We also developed a boundary integral formulation to simulate a red blood cell (RBC) squeezing through a submicron slit underprescribed inlet and outlet pressures. The main application of this computational study is to investigate splenic filtrations of RBCs and the corresponding in vitro mimicking microfluidic devices, during which RBCs regularly pass through inter-endothelial slits with a width less than 1.0μm. The diseased and old RBCs are damaged or destroyed in this mechanical filtration process.
Huijie Lu and Zhangli Peng. Boundary integral simulations of a red blood cell squeezing through a submicron slit under prescribed inlet and outlet pressures, Physics of Fluids,31:031902, 2019 and featured as Editor’s choice. pdf
Primary cilium: modeling of an organelle
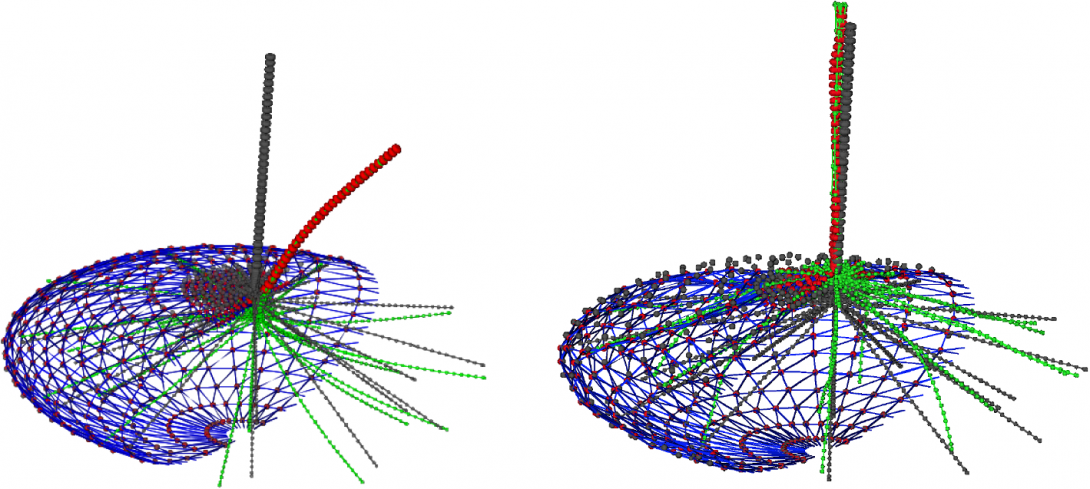
The fluctuating position of an optically trapped cilium tip under untreated and Taxol-treated conditions was used to characterize mechanical properties of the cilium axoneme and its basal body by combining experimental, analytical, and computational tools. We provide, for the first time, evidence that the persistence length of a ciliary axoneme is length-dependent; longer cilia are stiffer than shorter cilia. We demonstrate that this apparent length dependence can be understood by a combination of modeling axonemal microtubules as anisotropic elastic shells and including actomyosin-driven stochastic basal body motion. Our results also demonstrate the possibility of using observable ciliary dynamics to probe interior cytoskeletal dynamics. It is hoped that our improved characterization of cilia will result in deeper understanding of the biological function of cellular flow sensing by this organelle.
Justin Flaherty, Zhe Feng, Yuan-Nan Young, Zhangli Peng, and Andrew Resnick. Primary cilia have a length-dependent persistence length. Biomechanics and Modeling in Mechanobiology. 2019. DOI: 10.1007/s10237-019-01220-7.
DNA: modeling of a biomolecule
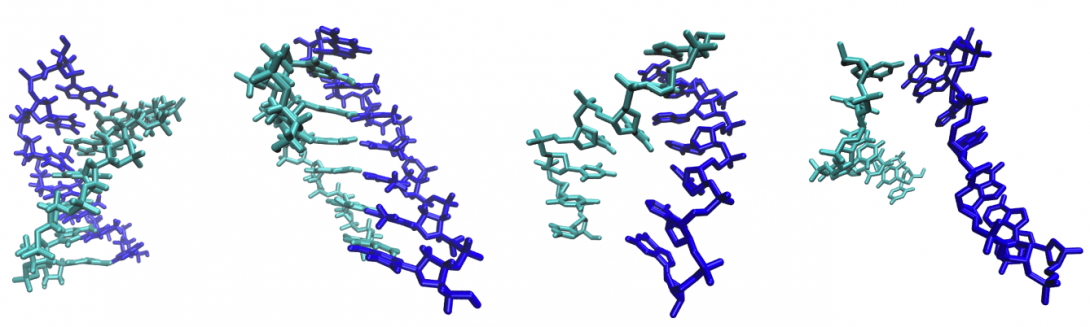
By treating DNA as a vibrating nonlinear lattice, an activated kinetic theory for DNA melting isdeveloped to capture the breakage of the hydrogen bonds and subsequent softening of torsional andbending vibration modes. With a coarse-grained lattice model, we identify a key bending mode withGHz frequency that replaces the hydrogen vibration modes as the dominant out-of-phase phononvibration at the transition state. By associating its bending modulus to a universal in-phase bendingvibration modulus at equilibrium, we can hence estimate the entropic change in the out-of-phasevibration from near-equilibrium all-atom simulations. This and estimates of torsional and bendingentropy changes lead to the first predictive and sequence-dependent theory with good quantitativeagreement with experimental data for the activation energy of melting of short DNA molecules withoutintermediate hairpin structures.
Sebastian Sensale, Zhangli Peng, and H.Chia Chang. Acceleration of DNA Melting Kinetics Using Alternating Electric Fields. Journal of Chemical Physics,149(8):085102, 2018. pdf
Sebastian Sensale, Zhangli Peng and H.-C. Chang. Kinetic theory for DNA melting with vibrational entropy, Journal of Chemical Physics, 147:135101. 2017.pdf
Arterial wall: modeling of a tissue
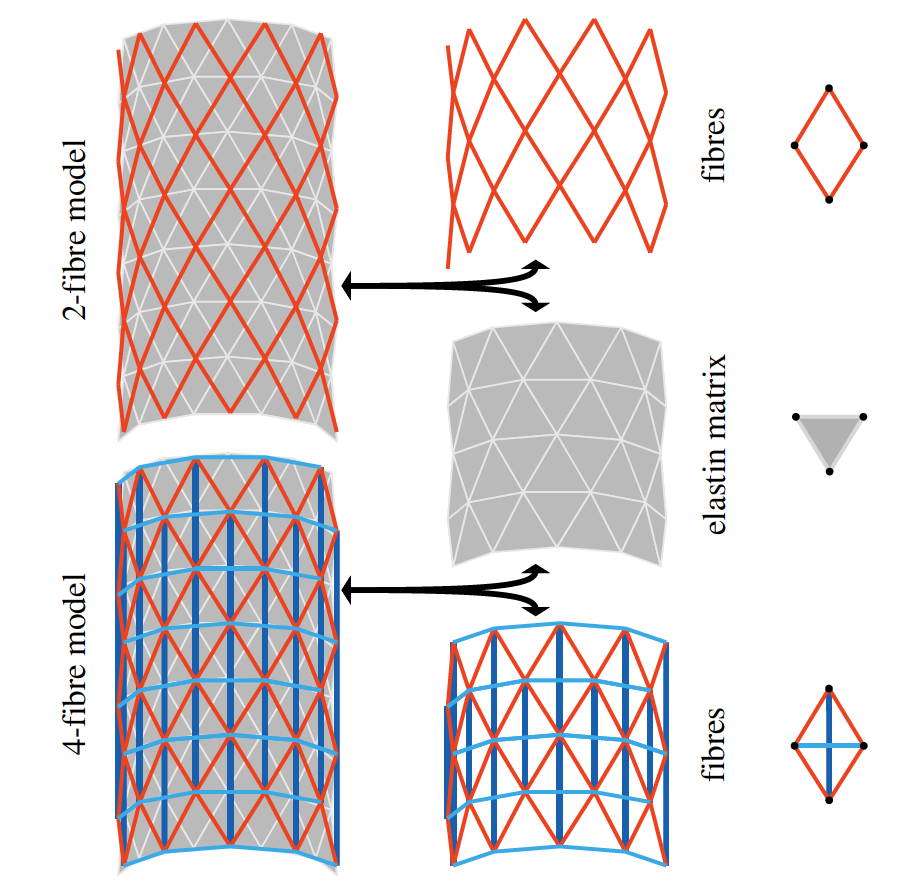
Blood vessels have unique properties that allow them to function together within a complex, self-regulating network. The contractile capacity of the wall combined with complex mechanical properties of the extracellular matrix enables vessels to adapt to changes in haemodynamic loading. Homogenized phenomenological and multi-constituent, structurally motivated continuum models have successfully captured these mechanical properties, but truly describing intricate microstructural details of the arterial wall may require a discrete framework. Such an approach would facilitate modelling interactions between or the separation of layers of the wall and would offer the advantage of seamless integration with discrete models of complex blood flow. We present a discrete particle model of a multi-constituent, nonlinearly elastic, anisotropic arterial wall, which we develop using the dissipative particle dynamics method. Mimicking basic features of the microstructure of the arterial wall, the model comprises an elastin matrix having isotropic nonlinear elastic properties plus anisotropic fibre reinforcement that represents the stiffer collagen fibres of the wall. These collagen fibres are distributed evenly and are oriented in four directions, symmetric to the vessel axis. Experimental results from biaxial mechanical tests of an artery are used for model validation, and a delamination test is simulated to demonstrate the new capabilities of the model.
Alexandra Witthoft, Alireza Yazdani, Zhangli Peng, Chiara Bellini, Jay D. Humphrey and George Em Karniadakis. A discrete mesoscopic particle model of the mechanics of a multi-constituent arterial wall. Journal of The Royal Society Interface, , 2015 pdf
Acoustic separation of circulating tumor cells (CTCs): modeling of microfluidics
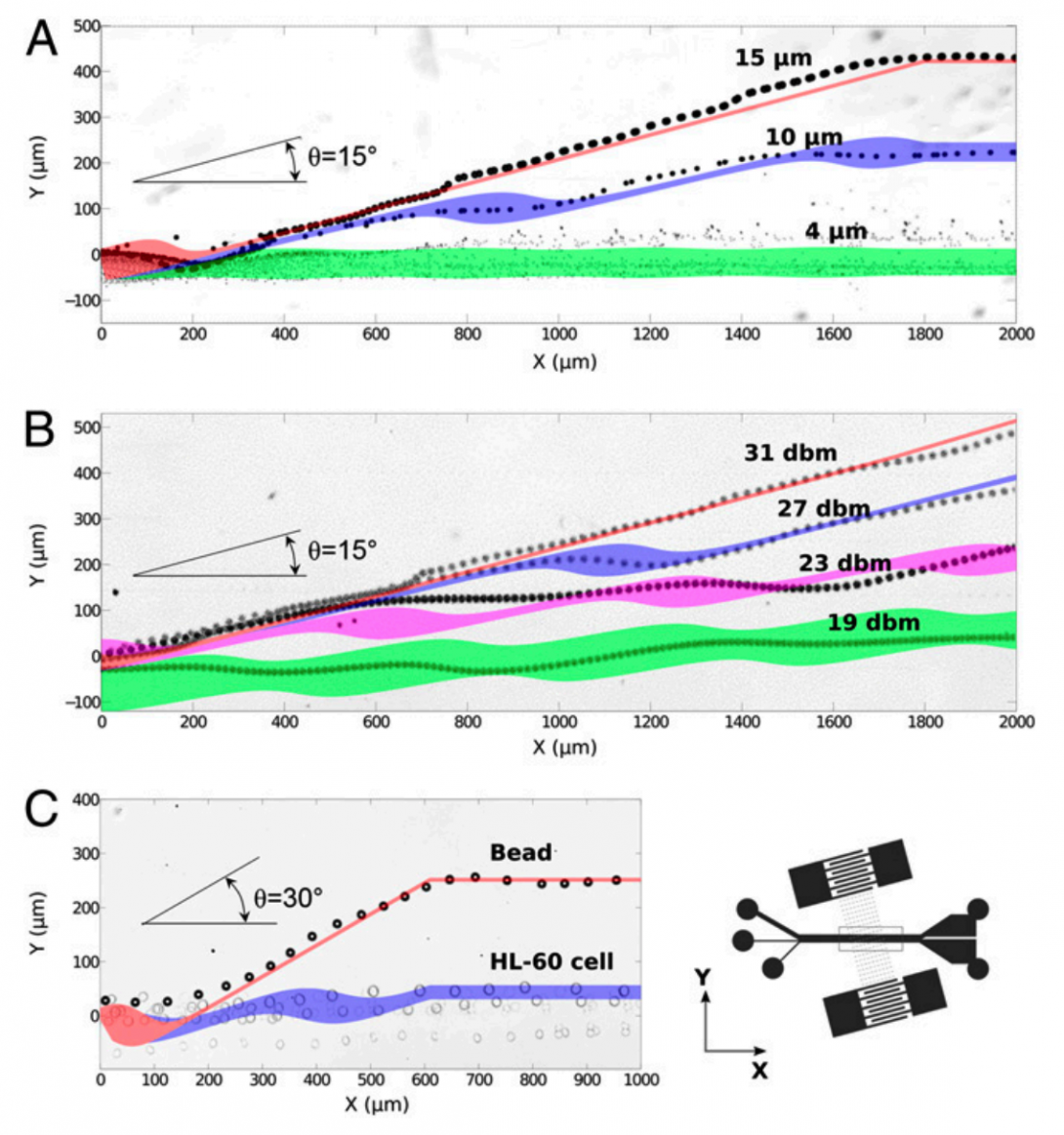
Separation of cells is a critical process for studying cell properties, disease diagnostics, and therapeutics. Cell sorting by acoustic waves offers a means to separate cells on the basis of their size and physical properties in a label-free, contactless, and biocompatible manner. The separation sensitivity and efficiency of currently available acousticbased approaches, however, are limited, thereby restricting their widespread application in research and health diagnostics. In this work, we introduce a unique configuration of tilted-angle standing surface acoustic waves (taSSAW), which are oriented at an optimally designed inclination to the flow direction in the microfluidic channel. We demonstrate that this design significantly improves the efficiency and sensitivity of acoustic separation techniques. To optimize our device design, we carried out systematic simulations of cell trajectories, matching closely with experimental results. Using numerically optimized design of taSSAW, we successfully separated 2- and 10-μmdiameter polystyrene beads with a separation efficiency of ∼99%, and separated 7.3- and 9.9-μm-polystyrene beads with an efficiency of ∼97%. We illustrate that taSSAW is capable of effectively separating particles–cells of approximately the same size and density but different compressibility. Finally, we demonstrate the effectiveness of the present technique for biological–biomedical applications by sorting MCF-7 human breast cancer cells from nonmalignant leukocytes, while preserving the integrity of the separated cells. The method introduced here thus offers a unique route for separating circulating tumor cells, and for label-free cell separation with potential applications in biological research, disease diagnostics, and clinical practice.
Peng Li, Zhangming Mao, Zhangli Peng, Lanlan Zhou, Yuchao Chen , Po-Hsun Huang , Cristina I. Truica, Joseph J. Drabick, Wafik S. El-Deiry, Ming Dao, Subra Suresh, and Tony Jun Huang. Acoustic Separation of Circulating Tumor Cells. Proceedings of the National Academy of Sciences of the U.S.A. 112:4970–4975.2015 pdf MIT News, Youtube
Xiaoyun Ding*, Zhangli Peng*, Sz-Chin Steven Lin, Michela Geri, Sixing Li, Peng Li, Yuchao Chen, Ming Dao, Subra Suresh and Tony Jun Huang. Cell Separation Using Tilted-Angle Standing Surface Acoustic Waves. Proceedings of the National Academy of Sciences of the U.S.A., (* contribute equally). pdf, youtube, MIT news
US. Patent: Separation of low-abundance cells from fluid using surface acoustic waves
https://patentimages.storage.googleapis.com/cd/69/41/12ca795444cb2e/US20170232439A1.pdf
DNA translocation through a nanopore: modeling of nanofluidics
![(A) Space charge density ? evaluated at the surface of the cylinder from FEM simulations as a function of the theoretical ?(?)/??. Ionic strengths were varied from 0.1 mM to 1 M and external field amplitudes were varied from 8 kV/m to 8 V/nm. Σ = 0.0275 C/m , nm, 2 ? = 1.185 ? = 11.22 nm and ? was evaluated at ? = ?/2. (B) Normalized screening charge ??/?? surrounding the cylinder (? ∈ [0,?]) obtained from FEM simulations (circles) for ? ∈ {1.185,2.37,5.925} nm, Σ ∈ {0.0275, 0.055,0.11,0.22} C/m and ionic strengths and external field amplitudes 2 correspond to the diffusion-layer scaling regions of (A). The dotted line is our high Peclet estimate from (4). Triangles represent the normalized screening charge surrounding a double stranded DNA molecule with 22 base-pairs from molecular dynamics (MD) simulations for molar strengths 0.01, 0.05, 0.1, 0.5 and 1 M and external fields ?∞ from 0.01 to 1 V/nm](https://peng.lab.uic.edu/wp-content/uploads/sites/568/2019/09/nanopore-1090x443.png)
We report a theory for biphasic ionic current signals during DNA and nanoparticles translocation through a solid-state nanopore that produces scaling results consistent with finite element simulations (FEM), molecular dynamics (MD) simulations and experiments. For standard nanopores designed for potential rapid sequencing applications, the electric field is enhanced by orders of magnitude due to field focusing and can severely deform the ion-cloud around the charged DNA. Highly fore-aft asymmetric space charge distribution leads to a universal quasi-steady comet-like structure with a long tail. In contrast to prior biphasic theories, the charge density and length of the tail, which are responsible for the negative resistive pulse, are shown to depend sensitively on the dimensionless applied field, the Peclet number Pe, with a ∓ 1 scaling, due to a balance between tangential migration and normal diffusion. An optimum Pe is predicted where the negative pulse has the maximum amplitude.
Sebastian Sensale, Zhangli Peng, and H.Chia Chang. Biphasic Signals during Nanopore Translocation of DNA and Nanoparticles due to Strong Ion Cloud Deformation. Nanoscale, Accepted. pdf
Flapping foils: modeling of finite-Reynolds-number flows
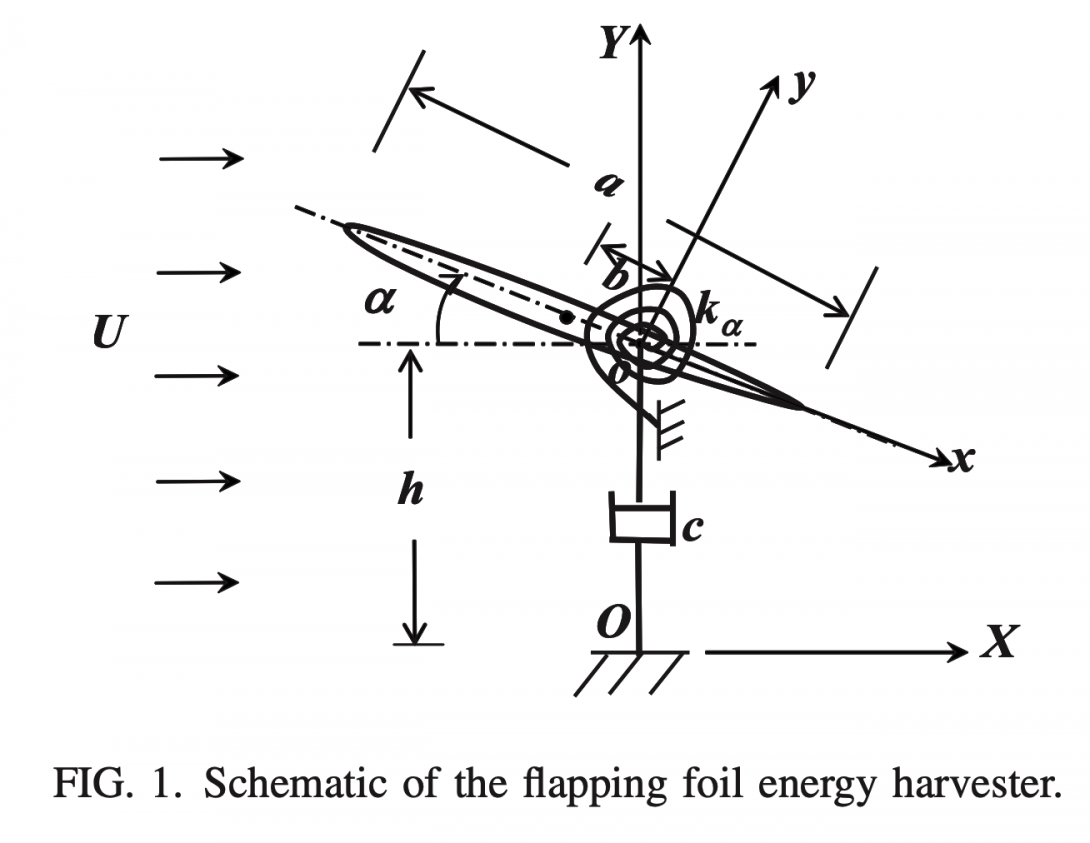
By using a Navier–Stokes model, we examine a novel flow energy harvesting device consisting of a flapping foil mounted on a damper representing the power generator and a rotational spring. The feasibility of our concept was recently experimentally proved by
We also used a two-dimensional computational hydrodynamic model to investigate the propulsive performance of a flapping foil system in viscous incompressible flows, which consists of two antiphase flapping foils in side-by-side arrangement.
Yan Bao, Dai Zhou, J.J. Tao, Zhangli Peng, H.B. Zhu, Z.L. Sun, and H.L. Tong, Dynamic interference of two anti-phase flapping foils in side-by-side arrangement in an incompressible flow. Physics of Fluids, 29:033601, 2017. pdf
Zhangli Peng and Qiang Zhu. Energy harvesting through flow-induced oscillations of a foil. Physics of Fluids, 21: 123602, 2009. pdf
Qiang Zhu and Zhangli Peng. Mode coupling and flow energy harvesting by a flapping foil. Physics of Fluids, 21: 033601, 2009. pdf
Inertial focusing: modeling microfluidics with finite-Reynolds-number flows
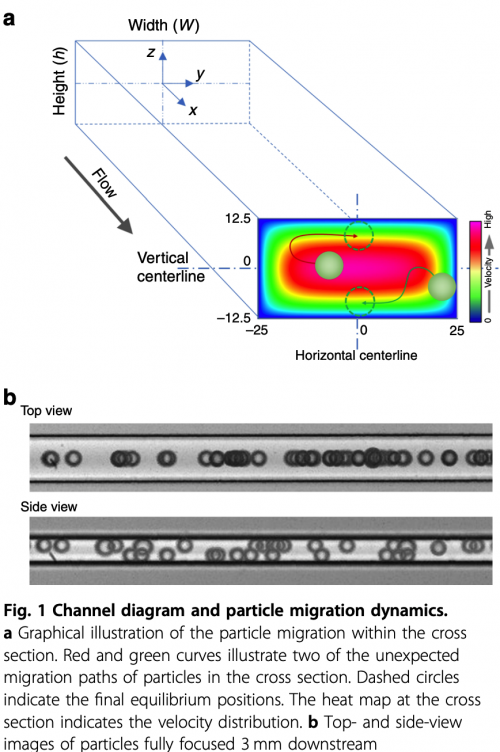
By using finite element method (FEM) and smoothed particle hydrodynamics (SPH), we are modeling inertial and elasto-inertial focusing of particles in microfluidics. This project is collaboration with Dr. Ian Papautsky group at UIC. We are focusing on understanding the focusing mechanisms and transient dynamics of particles in elasto-inertial flows and studying the effects of particle shape and deformability.
Jian Zhou, Zhangli Peng, Ian Papautsky. Mapping inertial migration in the cross-section of a microfluidic channel with high speed imaging. Accepted, Microsystems & Nanoengineering. 6:105, 2020 pdf